Covid-19 vaccination, first payment of 10 million USD about to be made for the Chinese vaccine
- 10 million USD, the Albanian Government approves the first payment for the Turkish distributor of Sinovax, made by the People’s Republic of China.
- Turkish Distributor announces lack of supply of Chinese made and packaged vaccines.
- Purchase Agreement changed. The Albanian Government agrees to the Chinese Vaccine packaged in Turkey, Coronavac 600SU/IM- Suspension.
- 14 April 2021, the Government allocates an additional 2 billion Albanian leke from its Covid-19 Vaccine Reserve Fund.
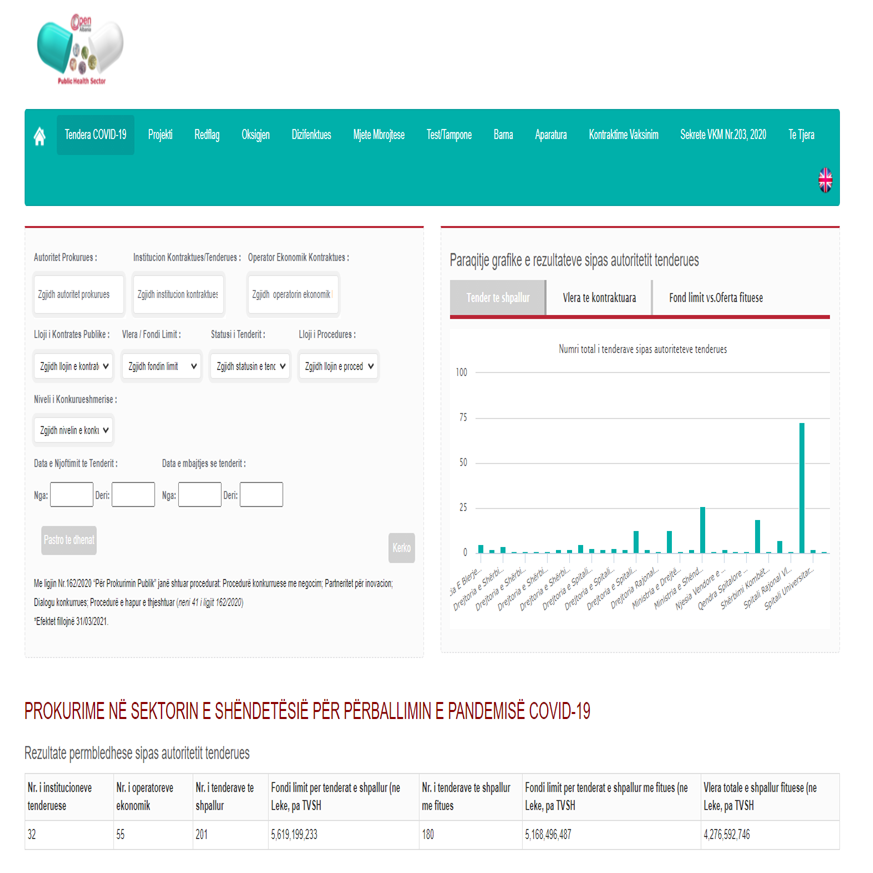
- Open Procurement Albania publishes all the acts on contracting, financial effects, and performance.
- The data on contracts related to Covid-19 are published in the framework of AIS Project “Contracting in a State of Natural Disaster Covid19 – Strengthening Independent Monitoring Mechanisms”
On 22 March 2021, the Council of Ministers issued Normative Act No. 9, approving the text of the Agreement between the Albanian State: Ministry of Health and Social Protection; Ministry of State for Reconstruction and Public Health Institute and the Authorized Distributor, the Turkish company Keymen Ilaç Sanayi Ve Ticaret A.Ş.
Scope of Agreement: Provide the Republic of Albania with the Vaccine against Covid-19 (Vero Cell) Inactivated Coronavac, created by Sinovac Life Science Co Ltd. and produced in the People’s Republic of China.
Article 3 of the Normative Act requires the Institutions and Parties not to publish the text of the agreement and its financial effects. Secret Confidential Agreement. According to a report published on the website of the Assembly of Albania attached to the Normative Act, the Committee of Experts for Immunization (an advisory body of the Minister of Health) examined the vaccine inactivated against Covid-19, Coronavac, created by Sinovac Life Science, Beijing, China.
The Committee concluded they were in favour of this vaccine. In their report, they say: The results of the first and second phase of this vaccine in China are published on the Lancet magazine, on 17 November 2020. This vaccine has already started the third phase trials in July 2020 in China, and later in Brazil, Turkey, Indonesia, and Chili. The third phase trials made in Brazil and Turkey assessed the vaccine efficiency on health employees offering treatment to COVID-19 patients. Currently, CoronaVac is approved for emergency use in China by the Regulatory Authority (Administration of national medical products), and approved for use in Azerbaijan, Bolivia, Brazil, Dominican Republic, Ecuador, Hong Kong, Indonesia, Laos, Mexico, Paraguay, Philippine, Thailand, Tunisia, Turkey, Ukraine, and Uruguay, etc. This vaccine is also used in EU Member states like Hungary.
The Council of Ministers approved the Normative Act No. 10, dated 24.03.2021 “Agreement between the parties”.
Financial Effects
This normative act has financial effects, which shall be covered by the state budget reserve fund.
The Law on the State Budget is amended three times from its approval until April 2021. The Normative Act No. 8, dated 22.3.2021 – the Reserve Fund is estimated to be about 5 billion leke (It was 4 billion according to the State Budget Law of 2021, so it is increased by 1 billion).
The purpose of the approval of this normative act is to secure the necessary funds through changes to the budgetary expenses. i.e. a reallocation between two elements of the reserve fund for purchasing the vaccine from the Turkish company “Keymen Ila9 Sanayi ve Ticaret”, A.S. created by Sinovac Life Science Co, Ltd. And produced in the People’s Republic of China. The total amount is 10 000 000 (ten million) USD, converted into leke by the official exchange rate on the date of payment, it is about 1.1 billion leke.
The Public Health Institute is the institution that will pay for the vaccine doses.
The normative act makes only one change to the budgetary expenses, a reallocation between two elements of the reserve fund, namely:
- 10 million USD, the Albanian Government approves the First Payment to the Turkish Distributor of Sinovax, produced in the People’s Republic of China.
By Decision of the Council of Ministers No. 174/2021, it was decided that the Institute of Public Health shall make a payment of 10 million USD (ten million American dollars) in favour of theauthorized distributor “Keymen Ilaç Sanayi ve ticaret” A.S., for supplying the Republic of Albania with the vaccine against COVID-19 (vero cell) inactivated, Coronavac, created by Sinovac Life Science Co, Ltd. And produced in the People’s Republic of China (point 1 of the decision). Point 2 of the decision provides that the ministry of Health and Social Protection is allocated the equivalent amount in Albanian lekë (as per the official exchange rate on the date of payment) of the 10 million USD in its budget approved for 2021, respectively to the Institute of Public Health, the economic account 6050000 (Product 91305AH) of the public health program (07450). This fund, according to pint 2 of this decision shall be covered by the reserve fund of the state budget, approved for 2021.
In its first parliamentary session following the suspension of work because of the electoral campaign, on May 4th, the Assembly’s agenda included the Consent to be reached on the two Normative Acts related with additional purchases of anti-Covid vaccines and their financial effects.
- The Turkish Distributor announces it can not supply Chinese-made and packaged vaccines.
Through Normative Act No. 14, dated 7.4.2021 – On changes and additions to Normative Act No. 9, dated 22.03.2021, the title and scope of the Agreement between the Parties is amended.
The accompanying report clarifies that the distribution company has announced that the Chinese Sonovac Life Sciences has stopped providing supplies of its product CoronaVac Covid 19 – Vero Cell as a product that is no longer available. After that, a Turkish company (distributor) has received supplies of Coronavac 600SU/IM- Suspension for injection packaged in puncture bottles of 0,5 ml, which contains the active substance, the inactivated viral antigen ‘Sars-CoV-2’, developed by SinoVac Life Science Co., Ltd., produced in the People’s Republic of China, and packaged in the Republic of Turkey by Keymen. Therefore, the Albanian party is proposed to continue getting Turkish-packaged supplies.
- Changes made to the Purchase Agreement, the Albanian State agrees to the Chinese vaccines packaged in Turkey Coronavac 600SU/IM- Suspension.
The Albanian party, after consultations with the Technical Committee of Immunization (meeting of 6 April 2021), decided to consider the same effects and agree to the proposal of the contracting party.
- 14 April 2021, the Government allocates an additional 2 billion Albanian lek to the State Budget for the Reserve Fund for Covid-19 related Vaccines and Situation.
Regarding its financial effects, a Consent is also to be reached on the Normative Act No. 18, dated 14.4.2021 on the first working day of the Assembly after the elections. Through this Act, the State Budget Reserve Fund is increased from 5 billion to 7 billion Albanian leke, i.e. an additional amount of 2 billion Albanian lekë, for covering the purchase of vaccines during 2021, including also any other eventual expenses of the Government related with the COVID-19 pandemic. The Albanian party has received information from formal exchanges with the Turkish Health Ministry about this vaccine being used in the Republic of Turkey.
Data on contracts signed in relation to Covid-19 are published in the framework of AIS Project on Contracting in a State of Natural Disaster Covid19 – Strengthening Independent Monitoring Mechanisms.All the contracts signed by the Albanian State for Supplies related with Covid-19 are available on the Open Procurement Albania database.